Which Statement Describes a Chemical Reaction at Equilibrium
The concentrations of reactants and products are equimolar. Which of the following statements best describes a system at chemical equilibrium.

16 6 Equilibrium Practice Test
Which statement describes a chemical reaction at equilibrium.

. 2 The reactants are completely consumed in the reaction. The kinetic energy of the system is zero. The concentrations of the products and reactants are constant.
No changes will be apparent as the forward in reverse reactions continue. Matter cannot be created or destroyed in a chemical reaction B. The rate of the forward reaction is greater than the rate of the reverse reaction.
1 the products are completely consumed in the reaction. Mar 15 2022. 1 The concentrations of the products and reactants are equal.
For any chemical reaction to be at equilibrium the rate of formation of the products must equal the rate of formation of the reactants such that at equilibrium their mass remains constant. 1 The products are completely consumed in the reaction. Use the dissociation reaction to answer the question.
3 The concentrations of the products and reactants are equal. 3 the same and the reaction has stopped. Reactants will convert to products The Gibbs free energy has reached a minimum The forward reaction rate is not equal to the reverse reaction rate O The concentration of product is equal to the concentration of reactant.
3 by adding water h2O. Which statement describes a chemical reaction at equilibrium. A small value K 1 indicates that the equilibrium reaction lies to the left in favor of reactants.
The appearance Will continue to change as the forward reaction continues at a slow balanced rate. Reactants and products are consumed and formed at the same rate. 4 The rate of the forward reaction is greater than the rate of the reverse reaction.
Which statement best describes the law of conservation of mass. The concentrations of the products and reactants are equal. Aug 2006-19 A chemical reaction is at equilibrium.
Which of the following statement describes a reversible chemical reaction that has reached equilibrium. Reactants in a chemical reaction rearrange to form a new substance or substances C. The principle relating the balanced chemical equation of a reversible reaction to its mass action expression or equilibrium constant expression Mass Action Expression.
3 The rate of the forward reaction is less than the rate of the reverse reaction. Both forward and reverse reactions have stopped. 2 The concentrations of the products and reactants are constant.
Molecules of reactants and products collide with the same frequency. 1 The rate of the forward reaction equals the rate of the reverse reaction. 2 faster and more product is produced.
The system consumes energy at a steady rate. The amounts of reactants and products do not change. A solution at equilibrium is yellow-orange.
Which statement describes a chemical reaction at equilibrium. FeSCN2 aq Fe3 aq SCN- aq In the reaction FeSCN2 ions are red Fe3 ions are pale yellow and SCN- ions are colorless. Reactants and products are consumed and formed at the same rate.
Chemistry questions and answers. The concentrations of reactants and products are equimolar. The system can do no work.
The rate of formation of the products must equal the rate of formation of the reactants. Which statement describes a chemical reaction at equilibrium. 2 the reactants are completely consumed in the reaction3 the concentrations of the products and reactants are equal4 the concentrations of the products and reactants are constant.
The yield of products is greater than 50. Two substances are dissolved in water and start to react which statement accurately describes the appearance of the solution when it reaches equilibrium. 4 The concentrations of the products and reactants are constant.
Which statement is always true for a chemical reaction that has reached equilibrium. Compared to the rate of the forward reaction the rate of the reverse reaction is. Le Chateliers Principle If a system is at equilibrium and any change is made to concentration pressure or temperature the reaction will shift to undo the effect of the stress and a new equilibrium is re-established.
4 the same and the reaction continues in both directions. Law of Mass Action. 1 faster and more reactant is produced.
Reactant molecules and product molecules have the same velocity. Molecules of reactants and products collide with the same frequency. The system releases energy at a steady rate.
Reactant molecules and product molecules have the same velocity. The concentration of SCN- ions is increased by a moderate amount. 4 The additional bromine ions cause the equilibrium to shift to the reactants.
Which statement describes a chemical reaction at equilibrium. 2 More liquid water molecules will change to water vapor until a new equilibrium is reached. Which statement correctly describes a chemical reaction at equilibrium.

Solved U Question 30 Consider The Following Reaction Chegg Com

Regents Chemistry Name Key Unit 10 Kinetics Practice Test Using

Solved I Which Statement Describes A Chemical Property Of Chegg Com

Tro Ic3 1 Increasing Temperature 2 Decreasing Temperature 3 Increasing Reactant Concentration 4 Increasing The Surface Area Of A Solid Reactant 5 All Of Ppt Download

Solved Which Of The Following Statements Describe A Reaction Chegg Com

Lab 13 Homework Questions Name Modified For Remote Chegg Com

9701 12 F M 19 March 2019 Mcq 12 A Level Chemistry Solved Mcq With Explanation The Chemistry Teacher

Solved I Which Statement Describes A Chemical Property Of Chegg Com
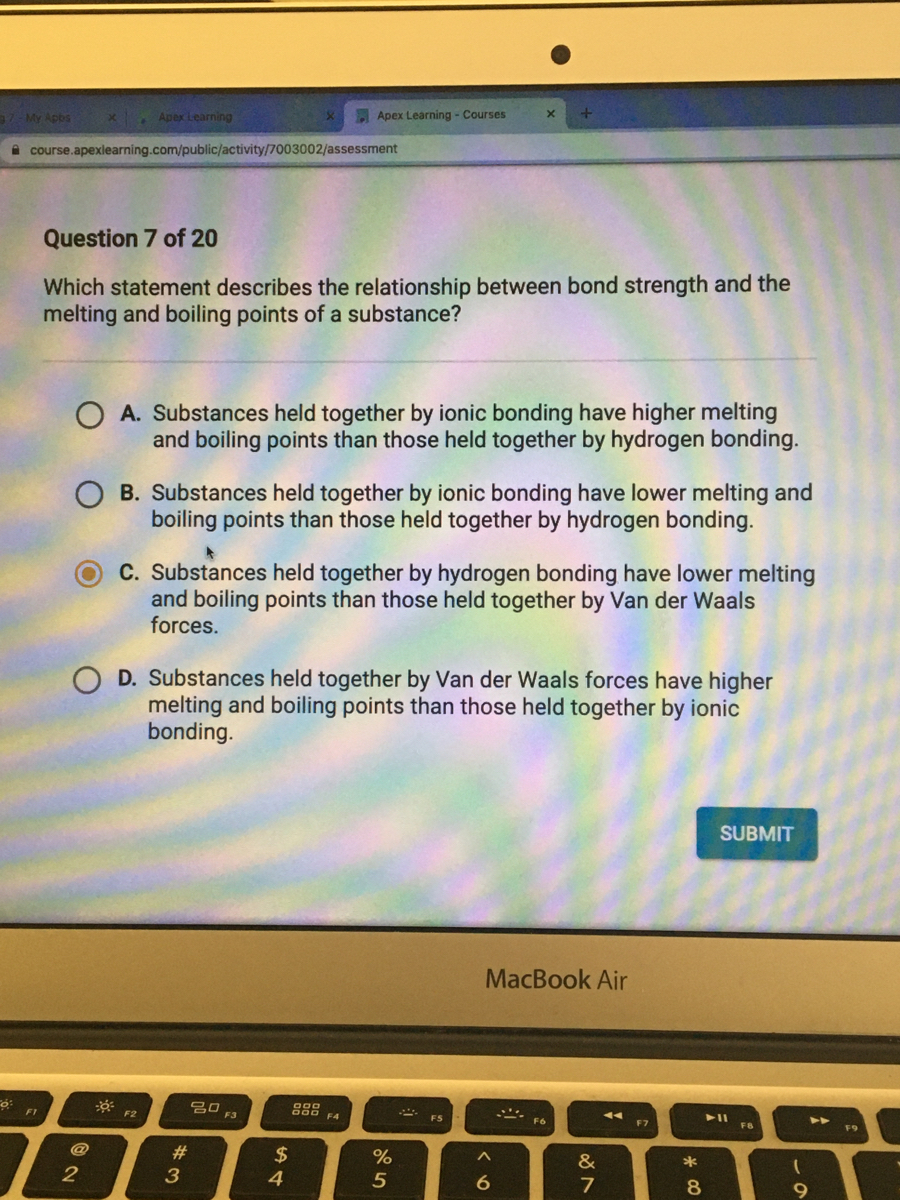
Answered Which Statement Describes The Bartleby

Vi Kinetics Equilibrium Ppt Download

Lab 13 Homework Questions Name Modified For Remote Chegg Com
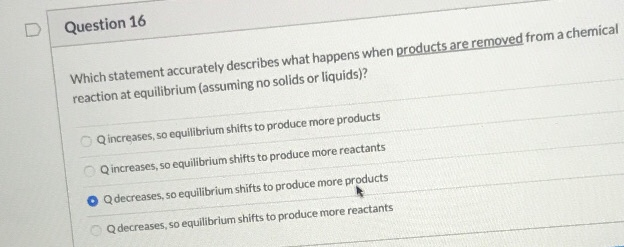
Solved Question 16 Which Statement Accurately Describes What Chegg Com
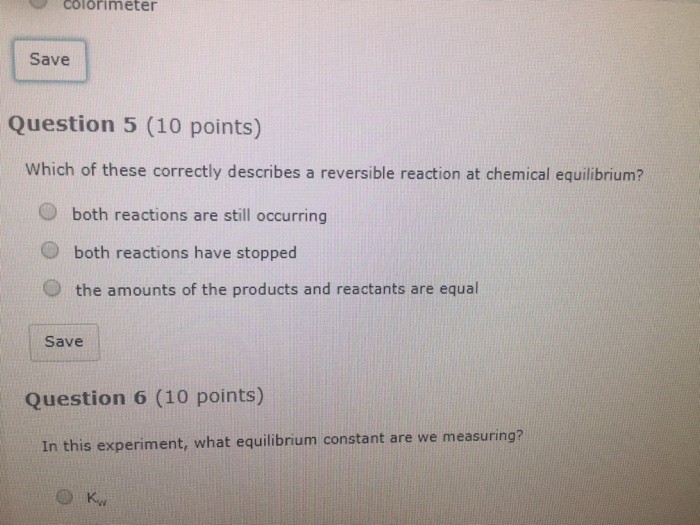
Solved Which Of These Correctly Describes A Reversible Chegg Com

What Is The Haber Process The Chemistry Journey The Fuse School Chemistry Teaching Chemistry Chemistry Education
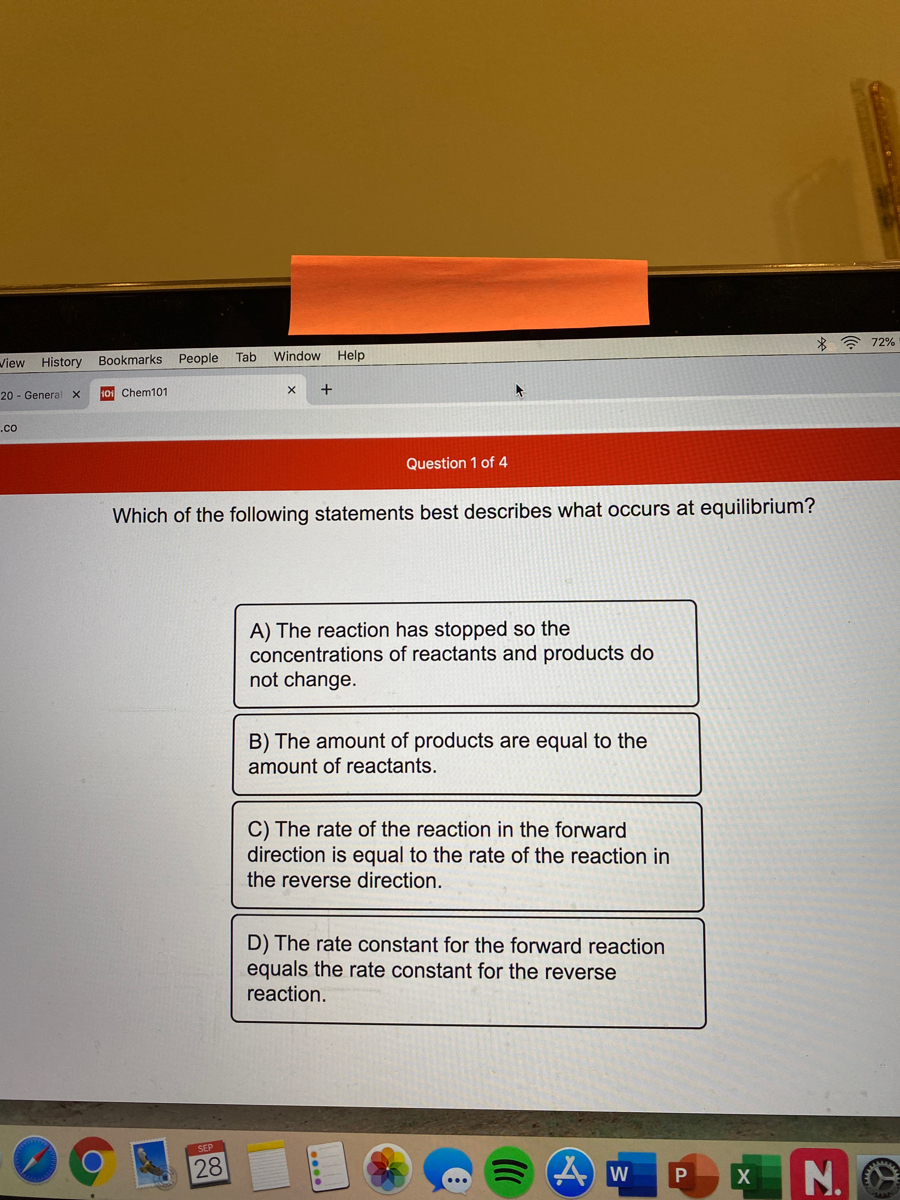
Answered Which Of The Following Statements Best Bartleby

Which Statement Describes A Reaction At Equilibrium Brainly In

Solved 1 Part A A Write A Balanced Chemical Equation That Chegg Com

Welcome To Learnapchemistry Com Ap Chemistry Question Paper Ap Chem

Solved Question 4 Which Of The Following Correctly Describes Chegg Com
Comments
Post a Comment